Originally posted on
http://www.jsieurope.org/safem/collect/safem/pdf/s2929e/s2929e.pdf
Section 02.
Of Blind Alleys and Things that Have Worked: History’s Lessons on Reducing Maternal Mortality
Wim Van Lerberghe1 and Vincent De Brouwere2
“It is only in recent years that the significance of the problem of maternal
mortality has been clearly realised by the health experts, …”
Monthly Epidemiological Report
Health Section, Secretariat,
League of Nations,
July 15th, 1930
Summary
As a matter of fact, the patterns of maternal mortality were very different during the 1870-1937 period in industrialised countries such as USA, England and Wales or Sweden. This chapter analyses the conditions under which the industrialised world has reduced maternal mortality over the last hundred years.
Preconditions appear to have been early awareness of the magnitude of the problem, recognition that most maternal deaths are avoidable, and mobilisation both of professionals and of the community.
Still, there have been considerable differences in the timing and speed of reduction of maternal mortality in different countries. These were related to the way professionalisation of delivery care was determined:
- first, by the willingness of the decision makers to take up their responsibility
- second, by the strategy adopted for making modern obstetrical care available to the population (and particularly by encouragement or dissuasion of midwifery care)
- and third, by the extent to which professionals were held accountable for addressing maternal health in an effective way
Where preconditions have been met and professionalisation of obstetric care has been adopted in developing countries, the same pattern of reduction of maternal mortality was observed, be the country still poor (Sri Lanka) or wealthier (Malaysia, Thailand).
Introduction
Where nothing effective is done to avert maternal death, “natural” mortality is probably of the order of magnitude of 1,500/100,000. Part of the world now has maternal mortality levels of the order of 5, some 300 times less.
Only rich industrialised countries have achieved such stable historical lows, but many middle-income and some poor countries are getting quite close, around or well below 50.
The majority of low-income countries, however, still has a long way to go. Poverty clearly contributes to this sorry state of affairs, but it does not explain everything.
Figure 1 shows that the estimated maternal mortality ratio in countries with a GNP of less than 1,000US$ ranges between 22 and 1,600. Even if one expresses GNP in purchasing power parity (PPP) terms, large inter-country differences remain: some still have maternal mortality ratios that are roughly equivalent to “natural” maternal mortality, others are way below.
For example, in the early 1990s Vietnam, Lesotho, the Central African Republic and Nepal all had a GNP-PPP between US$ 1,000 and 1,200. Their maternal mortality ratios estimates were 160, 600, 700 and 1,500. Certain countries definitely do better than others, even under severe poverty constraints.
These differences in track record get a different flavour if we compare present mortality levels in poor countries with those of industrialised countries at the beginning of the XXthcentury (Figure 2).
First, three out of four poor countries today have maternal mortality ratios that are higher than those of Sweden a century earlier, i.e. before caesarean section, blood transfusion or antibiotics, and at a time when nearly all deliveries took place at home. Lack of money for high-tech medicine is thus not the only explanation for the very high levels in some countries today.
Second, a century ago, there were major inter-country differences in the West – not as large as the disparities in the developing world today, but pretty impressive all the same. In the USA of 1900, for example, there were about 700 maternal deaths for 100,000 births, i.e. three times more than in Sweden.
Like in the developing world today, some of the industrialised countries have a better track record and managed to reduce maternal mortality much earlier than others.
2. @@@@@@
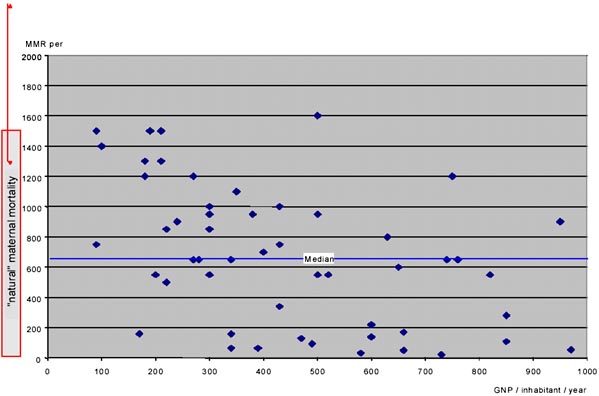
Figure 1. Maternal mortality ratios in the poorest countries, 1990 estimates. Ratios are plotted against the countries GNP and compared to “natural maternal mortality”. “Natural maternal mortality” is assumed to range between 1,100 and 2,500
SOURCES: GNP/inhabitant in 1993: World Bank 1995 Estimation of maternal mortality ratios in the 1990s: Stanton et al. 1995
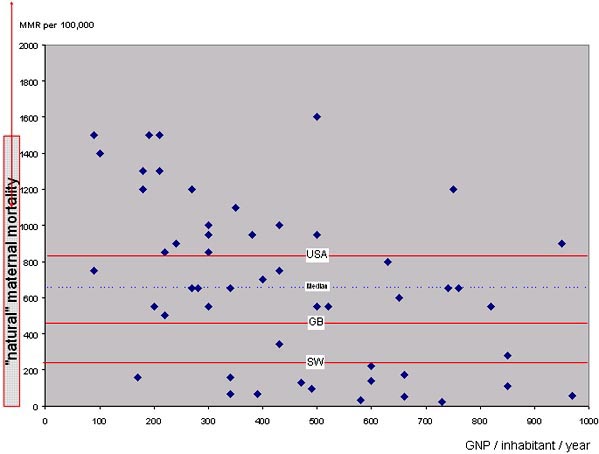
Figure 2. The same data as in figure 1, but compared to the 1900 maternal mortality ratios of the USA, England & Wales and Sweden
The history of these relative successes and failures is to a large extent a history of different approaches to the professionalisation of delivery care, even before technology-assisted hospital delivery became the norm3.
In order to make this case, we will look at differences in speed of reduction of maternal mortality in what is now the industrialised world, and attempt to identify the factors for success and failure. We will then look at obstacles faced by developing countries today, and draw some lessons with regard to strategic do’s and don’ts in maternal health policy.
Patterns of Reduction of Maternal Mortality in the West
Although the historical evidence is patchy, we do know that in countries like England maternal mortality levels were halved – compared to “natural maternal mortality” – towards the beginning of the XIXth century. Progress was in fact much more impressive for maternal than for overall mortality.
Loudon explains this “largely in terms of factors specific to childbirth rather than in terms of factors likely to have impinged on mortality of all causes”: “the decline in maternal mortality [between 1750 and 1850] was related both to an increasing proportion of midwife deliveries and to a higher standard of midwifery” (Loudon 1992a).
By 1850 maternal mortality was at a level of around 800/100,000 or even lower: levels not unlike the median poor country today.
Between the mid-XIXth century and the late 1930s, the patterns of reduction diverge markedly (Figure 3). On the one hand there are Northern European countries – Sweden is the prototype, but Denmark, Norway or the Netherlands follow roughly the same pattern: a clear downward trend from as early as 1870, stabilising at 250-300 per 100,000 between 1900 and 1940.
At the other extreme, maternal mortality ratios in the United States remained in a 600-800 bracket up to the mid-1930s. In-between the Swedish success story and the American failure one finds south-west Europe.
The North European success story is all the more impressive since it was achieved before modern hospital technology, transfusions, caesarean sections, or antibiotics became available, and, in the case of Sweden, in a poor rural country with a dispersed population.
The circumstances and strategies that made this possible and conversely, that caused the USA to lag behind, may help to understand why many poor countries have failed to reduce maternal mortality at a time when the technology to avoid maternal deaths is well known.

Figure 3. Maternal mortality from 1870 to 1993 in Sweden, the USA and England & Wales
Sweden @@@@@@ 3
Sweden
Sweden is unusual in the amount of historical information available on its demography. Its General Register has systematically collected individual health data since 1749. Very much in line with the development of vital statistics and quantitative methods in the XVIIIth century (Fox 1993), the Swedish Sundhetskommissionen reported that at least 400 women out of 651 dying in childbirth could have been saved if only there had been enough midwives (Högberg et al. 1986). This de facto introduced the notion of ‘avoidable maternal mortality’. The Sundhetskommissionen had enough authority to set a policy of training midwives in such numbers as to make sure that all deliveries – home deliveries, of course, were the norm – would be attended by qualified personnel.
Training large numbers of midwives was a slow and progressive process. Results were obtained only because authorities and professionals had a common purpose in tackling the problem of maternal mortality. One century after the report, in 1861, 40% of births were attended by certified professional midwives. The figure would double over the next four decades, to 78% in 1900. In the meantime the number of home deliveries assisted by traditional birth attendants dropped from 60% in 1861 to 18% in 1900. Only a small fraction of births, between 2% and 5%, took place in hospital4.
Midwives in Sweden were allowed to use forceps and hooks for craniotomy as early as 1829. They had a great deal of autonomy – in this thinly populated rural country that was a self-evident necessity – but were supervised by the local public health doctor. The latter could be called upon in case of major complication and was held accountable for official reports. The lines of authority were strong enough to generalise the introduction of aseptic techniques as early as 1881 – only a few years after it had been introduced in hospitals. The early adoption of this original combination of professional assistance to home deliveries and use of effective techniques enabled Sweden to achieve the lowest maternal mortality ratios in Europe (228 maternal deaths per 100,000 live births) by the beginning of the XXthcentury.
The Swedish success was partially a result of scientific and technical advances (Högberg et al. 1986) and partially a result of social changes empowered by public authorities. It is the combination of various ingredients that made this success possible (Figure 4). The potential of this recipe was further confirmed by later adopters of the same policy5 – i.e. the Netherlands, Denmark and Norway. An active policy of training midwives, selected for their social profile and capacity to introduce modernisation as ‘health missionaries’, with a close follow-up of compliance with hygiene and technical prescriptions (Marland 1997), reduced maternal mortality ratios to below 300 per 100,000 by 1920.
USA
The situation in the USA was quite different. Information became available only from 1900 onwards (Pearl 1921), much later than in Sweden, and there was no public policy to deal with what was not generally recognised as an issue. The debate was dominated by the (successful) attempts by obstetricians to marginalise midwifery (Declercq & Lacroix 1985, Borst 1988, King 1991, Reagan 1995).

Figure 4. The combination of technical and policy environment factors that made early reduction of maternal mortality in certain countries possible, and the obstacles in other countries
Midwives there were a mixed lot, going from the many untrained ‘neighbourhood midwives’ to the few highly trained midwives who were mainly recent European immigrants, but left to fend on their own, without support or supervision, despised and professionally isolated. Midwifery was actively discouraged by the lobby of obstetricians. “To the American obstetrician the midwife was ‘a relic of barbarism’ who must be abolished … If European countries persisted in employing midwives on a large scale, it only showed how backward Europe was compared to America.” (Loudon 1997).
In Sweden the notion of ‘avoidable maternal death’ had been used since the XVIIIth century and was at the basis of a public policy of midwifery coverage. In the USA this notion was essentially used by doctors for ‘scientific’ attacks on the market share of midwives (Fraser 1998). “Fear of the midwife’s real power, her ability to do the work of obstetrics – translated into a public portrayal of such women as primarily responsible for long labours and puerperal deaths. Physicians, by contrast, associated themselves with painless labour and safe childbirth” (Fraser 1998).
There was evidence that midwifery was a real alternative: where midwives were trained and supervised, as in Newark, they achieved remarkable results: a maternal mortality rate of 150 for midwife deliveries as opposed to 690 for deliveries by physicians (Loudon 1997). Nevertheless, obstetricians were left to effectively block the development of professional midwifery: by the 1920s this had already led to a decreasing pool of midwives in urban areas. In Richmond, for example, the midwife examining board had reduced the number of practising midwives from 105 to 47 in a 3-year period (Fraser 1998) and maternal mortality remained high.
The problem of maternal mortality only came on the policy agenda as a result of the public outcry against differences with Europe, in the early 1930’s. The first enquiries into maternal deaths, in New York in 1930-32 (Llewellyn-Jones 1974), led the New York Times to put the blame for avoidable maternal deaths on doctors (Porges 1985). Still, the medical lobby managed to ensure hegemony of hospital delivery. From the late XIXth century until today, the de facto policy was to promote institutional delivery by obstetricians. However, without mechanisms to guarantee access or quality standards, this failed to address the problem and actually contributed to mortality through iatrogenesis. The lack of norms and accessibility would only be offset by the Emergency Maternity Care Programme during the 2nd World-War (Schmidt & Valadian 1969).
England & Wales
In England and Wales women fared somewhat better than in the USA. Information had been available since the first half of the XIXth century. However, it was not until 1930 that the concept of ‘primary avoidable factor’ was identified and confidential investigations6 into maternal deaths were organised (Llewellyn-Jones 1974). Things then accelerated, and in 1932, the Ministry of Health sent a mission to Denmark, the Netherlands and Sweden to find out how these countries managed to achieve their low maternal mortality ratios (Oakley 1984).
The explanation had to do with the implementation of midwifery policies. Unlike Sweden, England & Wales had no active policy to generalise and professionalise midwifery before the XXth century, but the information had been available for quite some time and authorities were aware of the problem. Midwives were regulated with a ‘Midwives Act’ in 1902. In comparison with Sweden and other countries with the same level of technical knowledge, progress was slow. This was certainly due in part to the government’s indecision and to the fact that the funding of the necessary measures was left to the local authorities “who spent as little as possible on maternal and child health” (Loudon 1992a). The rampant competition between GP’s and midwives for access to the delivery-market compounded the insufficient funding (Mottram 1997). In a number of places – Manchester for example – the Medical Officers of Health implemented the Act correctly. Elsewhere, they did little or nothing. In districts where doctors were hostile to midwives, the act was often used “to harass midwives rather than to encourage their improvement” (Donnison 1988).
Accessible Technology and Reliable Hospitals
Between 1937 to 1970 maternal mortality dropped steeply throughout the industrialised world. By 1954 the USA and Sweden both reached 60 per 100,000. Further reductions after the 1970s brought maternal mortality ratios to the current low levels of less than 10. This new phase in the reduction of mortality ratios was a consequence of the improvement of techniques (antibiotics, caesareans, transfusions) in a context in which they were made available to the great majority of women, whether confined in hospital or at home. “Once the medical technology to treat obstetrical complications becomes widely accessible, it seems that the actual place of delivery is not of crucial importance.” (Maine et al. 1991). The improvement of financial access to professional care was a key factor, but health care systems also developed a culture of quality of care sustained by a system of control and professional accountability. This, in turn, was fed with information derived from generalised monitoring of mortality ratios and enquiries into maternal deaths.
Success or Failure: Combining the Right Ingredients
In the period before hospitals could intervene effectively and safely, the relative successes and failures appear to have depended less on the development of science and technology than on a combination of information, policy and strategy.
The first element was information. Countries with an early reduction in maternal mortality were also the ones where information on the extent of the problem had been around for a long time, and where public authorities reacted on this information. In countries where such information was more recent maternal mortality was not on the agenda, and the development of a control policy was delayed.
But the information was not enough. The nature of public administration, its commitment to public health and its capacity and willingness to react on information about avoidable deaths was just as important. What is sometimes less appreciated is that in the first half of the XXth century the debate on maternal mortality was not a matter for doctors and administrators alone. In various European countries, from the early XXth century to the late 1930s, committees concerned to improve maternal mortality were formed and associations with the same object, sometimes medical and sometimes lay, were founded. In the UK, for example, this eventually led to the 1938 Conference, attended by women from over 60 local associations, which gave rise to a comprehensive ‘Mothers’ Charter’ (Oakley 1984). In Sweden the concern of the medical establishment with the levels of maternal mortality was sufficient to obtain a public commitment. In many other countries legislation was only introduced and funds made available after pressure mounted from the civil society.
If information and public concern were elements that determined success or failure, another was the choice of policy. With hindsight we can say that before the technological hospital delivery of the second half of the XXth century came of age, the safer and more effective policy was to provide professional midwifery assistance at delivery, supervised, controlled, chosen on basis of a social profile that would promote modernisation (Marland 1997). Where this was the backbone of maternal health policies, mortality ratios dropped. Where it was not, they stagnated (Figure 5).
There is a whole body of evidence, and not only from north-west Europe, that shows that professional midwifery as such makes a difference, even in the absence of modern hospital technology. In the first half of the XXth century delivery was safer with a professional midwife than with a doctor. For example, Mary Beckenridge’s Frontier Nursing Service in the USA brought maternal mortality down to 66 in 1935-37 among the population it served, whereas in the same years hospital physicians in Lexington, Kentucky remained with a mortality of 800-900 among their white clientele.
Those countries that managed to get doctors to co-operate with a midwifery-based policy fared relatively well. Where doctors won the battle for professional dominance – and for their share of the market – women died. “It may be an extraordinary conclusion, but it is likely that [in the 1920s] at least 200,000 lives might have been saved by a maternity system based on trained midwives in the very country [the USA] in which the midwife was branded as a relic of the barbaric past” (Loudon 1997).
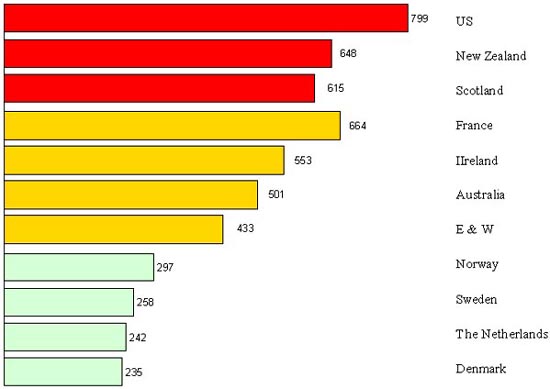
Figure 5. Maternal mortality in 1919-20 in countries with deliveries predominantly assisted by midwives (bottom), by both midwives and doctors (mid) and predominantly by doctors (top)
No country in the Western world has escaped the midwife-doctor debate, “from the violent denunciations of the midwife in the United States, through the struggles for midwife registration in Britain, to the more measured but none the less significant discussions on the place of Dutch midwives in providing obstetric care” (Marland & Rafferty 1997). But the potential of midwives has been realised only where they were well trained, supervised, regulated, and held accountable for results. The relatively poor performance of doctors and hospitals – and their contribution to mortality through iatrogenesis – in the same period can best be explained by the greater difficulty in making them adhere to scientific standards and in holding them accountable for results.
It was not, however, merely a question of public authorities making the right policy choice; it was also a matter of being able to implement such a policy with enough authority to make professional delivery care accessible. North-west Europe adopted different versions of Sweden’s strategy of putting ‘a midwife in every parish’: a strategy based on proximity, geographical, but also cultural and financial, based on a long term effort in financing and training as well as regulating midwifery. When hospital- and obstetrician-based delivery care came of age in the second half of the XXth century, proximity and access also became the determining factors, as in many developing countries today.
This combination of the technical and political factors (Figure 4) resulted in a significant reduction of the maternal mortality in Sweden, Japan, Denmark, Norway and The Netherlands, even without hospital technology. In countries like the USA, Belgium, Great-Britain, France or Italy ingredients were missing and mortality remained higher until modern hospital technologies became accessible; in those countries medicalisation of delivery would eventually be more pronounced. The commitment and sense of responsibility of health professionals and the State, clearer understanding of the causes of mortality – associated with the advent of effective technologies: caesarean section, antibiotics, blood transfusion – and extension of coverage to the population as a whole enabled the industrialised countries to attain extremely low maternal mortality ratios in some twenty years (between 1937 and 1960). By that time it did not make a difference whether the policy was to promote confinement in hospital (as in the United States) or at home (as in the Netherlands): that became a question of culture and efficiency, not of effectiveness in reducing mortality.
References
Backett M, Davies AM and Petros-Barvazian A (1984). L’approche fondée sur la notion de risque et les soins de santé. Notamment la santé maternelle et infantile (y compris la planification familiale). Genève: OMS.
Boerma JT and Mati JK (1989). “Identifying Maternal Mortality through Networking: Results from Coastal Kenya.” Stud.Fam.Plann. 20(5),245-253.
Borst CG (1988). The training and practice of midwives: a Wisconsin study. Bull. Hist. Med. 62:606-627.
Bouvier Colle MH, Varnoux N, Bréart G and Medical Expert Committee (1995). “Maternal deaths and substandard care: the results of a confidential survey in France.” Eur. J. Obstet. Gynecol. Reprod. Biol. 58, 3-7.
Browne FJ and Aberd (1932). “Antenatal care and maternal mortality.” Lancet (July 2),1-4.
Cook R (1989). “The role of confidential enquiries in the reduction of maternal mortality and alternatives to this approach.” Int.J.Gynecol.Obstet. 30, 41-45.
Cranch GS (1974). “The role of nursing/midwifery in maternal and infant care.” In: Nursing-midwifery aspects of maternal and child health and family planning, Washington: PAHO, p.30-34.
De Brouwere V & Van Lerberghe W (1998). Les besoins obstétricaux non couverts. Paris: L’Harmattan.
De Brouwere V, Tonglet R & Van Lerberghe W (1997). “La ‘Maternité sans Risque’ dans les pays en développement: les leçons de l’histoire” Studies in Health Services Organisation & Policy, 6, Antwerp, ITGPress.
Declercq E and Lacroix R (1985). The immigrant midwives of Lawrence: the conflict between law and culture in early twentieth-century Massachusetts. Bull. Hist. Med. 59,232-246.
Derveeuw M, Litt V, De Brouwere V & Van Lerberghe W (1999). “Too little, too late, too sloppy: delivery care in Africa”. Lancet 353, 409.
Donnison J (1988). Midwives and Medical Men: A History of the Struggle for the Control of Childbirth. Hienemann, London.
Duncan JM (1870). On the mortality of Children and Maternity Hospitals. Edinburgh, Adam and Charles Black.
EA2RS (1996). Mongolia: poverty assessment in a tranistion economy. World Bank.
Ebrahim GJ (1989). “Safe Motherhood in the 1990s”. J Trop Pediatr 935(6),272-273.
Filippi VG, Alihonou E, Mukantaganda S, Graham WJ and Ronsmans C (1998). “Near misses: maternal morbidity and mortality.” Lancet 351,145-146.
Fox DM (1993). “The medical institutions and the state” In Companion encyclopedia of the history of medicine, vol. 2 W. F. Bynum & R. Porter, eds., Routledge, London & New York, pp. 1204-1230.
Fraser GJ (1998). African American Midwifery in the South. Dialogues of birth, race, and memory. Harvard University Press, Cambridge Mass & London.
Godber G (1994). The origin and inception of the Confidential Enquiry into Maternal Deaths. Br. J. Obstet. Gynaecol. 101,946-947.
Graham WJ & Campbell OMR (1992). “Maternal health and the measurement trap.” Soc. Sci. & Med. 35,967-977.
Graham WJ, Brass W and Snow RW (1989). “Estimating maternal mortality: the sisterhood method.” Stud.Fam.Plann. 20 (3),25-135.
Hibbard BM, Anderson MM, Drife JO, Tighe JR, Gordon G, Willatts S et al. (1996). Report on Confidential Enquiries into Maternal Deaths in the United Kingdom 1991-93, London: HMSO.
Högberg U, Wall S and Broström G (1986). “The impact of early medical technology of maternal mortality in late XIXth century Sweden.” Int.J.Gynecol.Obstet. 24, 251-261.
Jaffré Y & Prual A (1994). Midwives in Niger: an uncomfortable position between social behaviour and health care constraints. Soc. Sci. Med. 38(8),1069-1073.
Jahn A, Razum O & Berle P (1998). Routine screening for intrauterine growth retardation in Germany: low sensitivity and questionable benefit for diagnosed cases. Acta Obstet Gynecol Scand. 77,643-648.
Jahn A (1999). Is the risk approach in antenatal care still justified? Lessons from Tanzania and Germany. Gynaecology Forum, 4(4),14-18.
Kasongo Project Team, Van Lerberghe W & Van Balen H (1984). “Antenatal screening for fetopelvic dystocias; a cost- effectiveness approach to the choice of simple indicators for use by auxiliary personnel”, J Trop. Med Hyg. 87,173-183.
King CR (1991). “The New-York maternal mortality study: a conflict of professionalization.” Bull. Hist. Med. 65 (3),476-502.
King M (1970). Medical care in developing countries: a symposium from Makerere, Nairobi: Oxford University Press, 309 pages.
Koblinsky MA, Campbell O & Heichelheim J (1999). Organizing delivery care: what works for safe motherhood? Bull. World Health Organ 77(5),399-406.
Kwast BE (1988). Unsafe motherhood: a monumental challenge. A study of maternal mortality in Addis Ababa, Leiderdorp, The Netherlands.
Langer A, Hernandez B, Garcia-Barrios C, Saldaña-Uranga GL and National Safe Motherhood Committee of Mexico (1999). “Identifying interventions to prevent maternal mortality in Mexico: a verbal autopsy study.” In: M. Berer and Sundari (eds) Safe Motherhood Initiatives: critical issues, 127-136.
Lawson B and Stewart DB (1967). Obstetrics and Gynaecology in the Tropics and Developing Countries, London: Edward Arnold.
Leng HC (1990). Health and Health Care in Malaysia. Institute for Advanced Studies Monograph Series SM 3. University Malaya.
Llewellyn-Jones D (1974). Human Reproduction and Society. London: Faber and Faber.
Loudon I (1992a). Death in childbirth. An international study of maternal care and maternal mortality 1800-1950 Oxford University Press, London.
Loudon I (1992b). “The transformation of maternal mortality.” BMJ 305,1557-1560.
Loudon I (1997). “Midwives and the quality of maternal care.” in: Marland H and Rafferty AM, Midwives, Society and Childbirth. Debates and controversies in the modern period. Routledge, London & New York, 180-200.
Mahler H (1987). “The Safe Motherhood Initiative: a call to action.” Lancet, 668-670.
Maine D, Rosenfield A, McCarthy J, Kamara A and Lucas AO (1991). Safe Motherhood Programs: options and issues, New-York: Columbia University.
Mangay-Maglacas A (1990). “Traditional birth attendants.” In: Health care for women and children in developing countries, edited by H. M. Wallace and K. Giri, Oakland (USA):Third Party Publishing Company, p. 229-241.
Marland H (1997). “The midwife as health missionary: the reform of Dutch childbirth practices in the early twentieth century”, In Marland H & A. M. Rafferty, eds.: Midwives, Society and Childbirth. Debates and controversies in the modern period., Routledge, London, pp. 153-179.
Marland H and Rafferty AM (1997). Midwives, Society and Childbirth. Debates and controversies in the modern period. Routledge, London & New York.
Maternal and Child Health Division Ministry of Health and Welfare of Japan and Mothers’ and children’s Health and Welfare Association (1992). Maternal and Child Health in Japan, Tokyo: Mothers’ and Children’s Health and Welfare Association.
Mora G & Yunes J (1993). Maternal mortality: an overlooked tragedy. In: Gender, women, and health in the Americas, pp. 62-79, ed E. Gómez Gómez, Washington: Pan American Health Organization.
Mottram J (1997). “State Control in Local Context. Public health and midwifery regulation in Manchester, 1900-1914.” in: Marland H and Rafferty AM, Midwives, Society and Childbirth. Debates and controversies in the modern period. Routledge, London & New York, 134-152.
Oakley A (1984). The capture womb. A history of the medical care of pregnant women. Basil Blackwell, Oxford.
OMS (1952). Comité d’experts de la maternité. Premier rapport. Etude préliminaire. SRT n°51, Organisation Mondiale de la Santé, Geneva, 28p.
Paxman JM, Rizo A, Brown L & Benson J (1993). The clandestine epidemic: the practice of unsafe abortion in Latin America. Stud. Fam. Plann. 24(4),205-226.
Pearl R (1921). “Biometric data on infant mortality in the United States Birth Registration Area, 1915-1918.” Am.J.Hyg. 1,419-439.
Porges RF (1985). “The response of the New-York Obstetrical Society to the report by the New-York Academy of Medicine on maternal mortality, 1933-34.” Am. J. Obstet. Gynecol. 152,642-649.
Prual A, Bouvier-Colle MH, de Bernis L & Breart G (2000). “Severe maternal morbidity from direct obstetric causes in West Africa: incidence and case fatality rates”, Bull. World Health Organ 78(5),593-602.
Rao KB (1981). “Maternity care.” In: Maternal and child health around the world, edited by H. M. Wallace and G. J. Ebrahim, London: The Macmillan Press Ltd, p. 76-81.
Reagan LJ (1995). Linking midwives and abortion in the progressive era. Bull. Hist. Med. 69,569-598.
Rendon L, Langer A & Hernandez B (1993). Women’s living conditions and maternal mortality in Latin America. Bulletin of PAHO 27(1),56-64.
Reynolds FN (1934). “Maternal mortality” Lancet (29 December):1474-1476.
Rochat RW (1981). “Maternal mortality in the United States of America”. Wld. Hlth. Statist. Quart. 34,2-13.
Ronsmans C, Vanneste A-M, Chakraborty J and van Ginneken JK (1998). “A comparison of three verbal autopsy methods to ascertain levels and causes of maternal deaths in Matlab”, Bangladesh. Int. J. Epidemiol. 27,660-666.
Rooney C (1992). Antenatal care and maternal health: how effective is it? A review of evidence, Geneva: World Health Organization.
Rosa FW (1981.) “The status of maternal and child care in developing countries.” In: Maternal and child health around the world, edited by H. M. Wallace and G. J. Ebrahim, London: The Macmillan Press Ltd, p. 3-16.
Rosenfield A & Maine D (1985). “Maternal mortality: a neglected tragedy. Where is the M in MCH?” Lancet 2 (446),83-85.
Rosenfield A (1989). “Maternal mortality in developing countries: an ongoing but neglected ‘epidemic’.” J.A.M.A. 262,376-379.
Schmidt WM & Valadian I (1969). “Maternal and child health activities.” In JJ Hanlon ed Principles of Public Health administration, The CV Mosby Company, Saint Louis, 367-381.
Seneviratne HR & Rajapaksa LC (2000). “Safe motherhood in Sri Lanka: a 100-year march”, Int.J.Gynaecol.Obstet. 70(1),113-124.
Smith JB, Coleman NA, Fortney JA, De-Graft Johnson J, Blumhagen DW & Grey. TW (2000). The impact of traditional birth attendant training on delivery complications in Ghana. Health Policy & Planning15(3),326-331.
Smith JS & Janowitz B (1984). “La surveillance prénatale.” In: Janowitz B, J Lewis, N Burton and P Lamptey Santé reproductive en Afrique Subsaharienne: les questions et les choix, USA: Family Health International, 19-22.
Stanton C, Hill K, AbouZahr C & Wardlaw T (1995). Modelling maternal mortality in the developing world. WHO & UNICEF, Geneva.
Stanton C, Abderrahim N & Hill K (2000). “An assessment of DHS maternal mortality indicators”. Stud.Fam.Plann. 31(2),111-123.
Strong TH Jr (2000). Expecting Trouble: The Myth of Prenatal Care in America, New York: New York University Press.
Suleiman AB, Mathews A, Jegasothy R, Ali R & Kandiah N (1999). “A strategy for reducing maternal mortality”, Bull.World Health Organ 77(2),190-193.
UNICEF (1998). Situation analysis of children and women in Iraq. Unicef, New York.
Van der Does CD and Haspels AA (1972). “Antenatal care.” In: C. D. Van der Does and A. A. Harpels, ed. Obstetrical and gynaecological hints for the tropical doctor, Utrecht: A. Oosthoek’s, 1-6.
Van Lerberghe W, de Béthune X & De Brouwere V (1997). “Hospitals in sub-Saharan Africa: why we need more of what does not work as it should?” Trop Med Int. Health 2(8),799-808.
Van Lerberghe W & De Brouwere V (2000). “Etat de santé et santé de l’Etat en Afrique subsaharienne”, Afrique Contemporaine 135: 175-190.
Van Lerberghe W (1993). “Les politiques de santé africaines: continuités et ruptures”, Bull Séances Acad R Sci Outre Mer. 39, 205-230.
Wessel H, Reitmaier P, Dupret A, Rocha E, Cnattingius S & Bergstrom S (1999). “Deaths among women of reproductive age in Cape Verde: causes and avoidability”, Acta Obstet.Gynecol.Scand. 78(3),225-232.
Walsh JA & Warren K (1979). “Selective Primary Health Care: an Interim Strategy for Disease Control in Developing Countries”, N.Engl.J.Med., 301:967-974.
WHO (1986). “Maternal mortality: helping women off the road to death.” WHO Chronicle 40(5),175-183.
WHO and UNICEF (1996). Revised 1990 estimates of maternal mortality. A new approach by WHO and UNICEF, Geneva: WHO.
Wibulpolprasert S ed. (2000). Thailand health profile 1997-1998. Ministry of Public Health. Bangkok, ETO Press.
World Bank (1995). World Development Report 1995. Workers in an integrating world. Oxford University Press, New-York.
Continue to
Part Two – Of Blind Alleys and Things That Worked — preventable maternal mortality